Abstract
Introduction:
Sporadic cases of patients with a combined T-cell malignancy and plasma cell dyscrasia (PCD) have been reported in the literature. While the most commonly observed association is with T-cell large granular lymphocytic leukemia (T-LGLL) and PCD, there are case reports of other T-cell malignancies and PCD, such as peripheral T-cell lymphoma (PTCL) and angioimmunoblastic T-cell lymphoma (AITL) with multiple myeloma (MM). Nearly one-third of MM patients develop clonal T-cell populations that share a similar immunophenotype to T-LGLL, suggesting likely under diagnosis of concomitant T-cell malignancies in patients with PCD. However, with the limited data available regarding the overlap between PCD and T-cell malignancy, the significance, associated pathogenesis, and impact on survival outcomes is unknown. The purpose of this study is to describe the outcomes of patients with overlapping T-cell malignancies and PCD in order to determine the survival outcomes and ultimately make management/diagnostic recommendations.
Methods:
In this IRB approved study, we retrospectively evaluated patients with concomitant T-cell malignancy and PCD at Ohio State University from 2010-2020. Patients were identified using a database search for T-cell malignancies as well as PCD. All patients that were included met the 2016 World Health Organization diagnostic criteria for their respective T-cell malignancy and PCD. Progression-free survival (PFS) was measured as time from the start of treatment until first progression, death, or last follow-up according to the Kaplan-Meier method with median survival times and 95% confidence intervals reported.
Results:
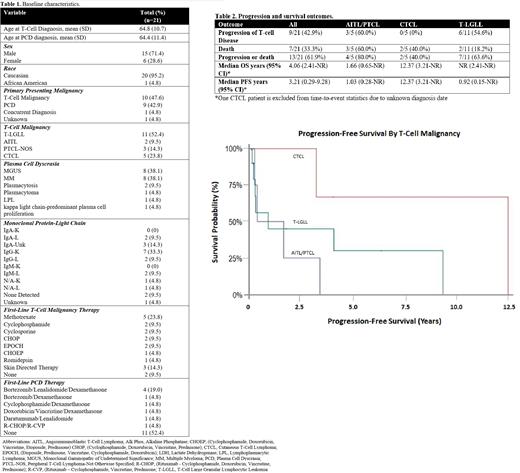
A total of 21 patients, with a median follow-up time of 22 months (range 1-153), were included in this analysis. Baseline demographics are in table 1. The most common T-cell malignancy was T-LGLL (11/21; 52%) and the most common PCD were MGUS (8/21; 38%) and MM (8/21; 38%). Ten (48%) patients presented with a T-cell malignancy as their primary malignancy, 9 (43%) presented with a PCD as their primary malignancy, 1 (5%) patient was diagnosed with both at the same time, and for 1 (5%) patient it is unknown. Within the cohort, 62% (13/21) of patients received primary treatment for their T-cell malignancy and 38% (8/21) of patients received primary treatment for their PCD. Of the 7 patients that had their PCD clone 4 were treated concomitantly and 3 were treated for only their T-cell malignancy. Overall, 9/21 (42.9%) of patients had progression of their T-cell malignancy. A summary of outcomes is provided in Table 2. 54.6% of patients with T-LGLL and 60% of patients with AITL/PTCL experienced progressive T-cell disease and no patients with CTCL had progressive T-cell disease. The median overall survival (OS) across all patients was 4.1 years. Median OS was not reached for patients with T-LGLL, 1.7 years for AITL/PTCL, and 12.4 years for CTCL. PFS was 11 months for patients with T-LGLL, 1 year for AITL/PTCL, and 12.37 years for CTCL. Survival probability is shown in Figure 1. The rates of progression, OS, and PFS were consistent with previously published data for patients with these T-cell malignancies.
Conclusions:
Herein, we characterize a cohort of patients with concomitant T-cell malignancies and PCD with an emphasis on survival outcomes. Our data suggests that there is no PFS or OS difference for patients with T-cell malignancies and concomitant PCD when treated with standard T-cell directed therapy. There is the potential that treating a patient's T-cell malignancy may lead to resolution of their PCD clone, even without therapy directed at the PCD. While our data is limited by small sample size, this report represents the largest data set available in this rarely described patient population. Larger retrospective cohort studies are needed to further characterize this population and validate these findings. For patients with T-cell malignancies as the primary diagnosis with concomitant PCD, treatment with standard T-cell directed therapies is recommended with continued follow-up and monitoring of the concomitant PCD.
Bumma: Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees. Porcu: Viracta: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Innate Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; Daiichi: Honoraria, Research Funding; Kiowa: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Spectrum: Consultancy; DrenBio: Consultancy. Brammer: Seattle Genetics: Speakers Bureau; Celgene: Research Funding; Kymera Therapeutics: Consultancy.